Continuous freeze-drying of mRNA LNPs enables storage at higher temperatures
Publications
Continuous freeze-drying of mRNA LNPs enables storage at higher temperatures
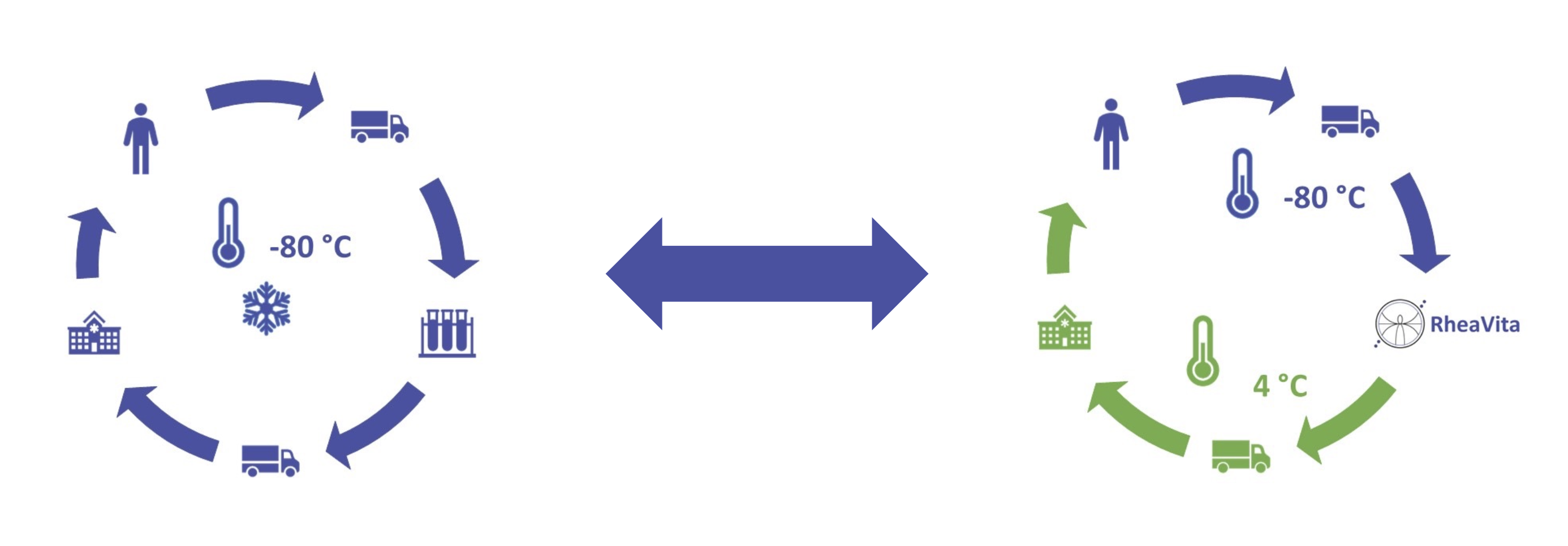
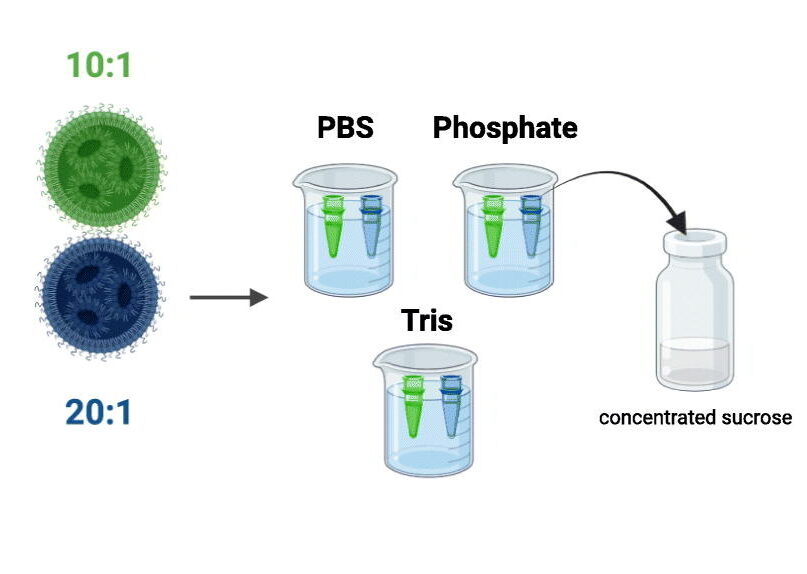
Research at Ghent University, in collaboration with The University of British Columbia, has shown that continuous freeze-drying of mRNA LNPs enables long-term storage at higher temperatures for months without losing their transfection properties.
The full article can be read and downloaded here
Please contact us via info@rheavita.com if you wish to learn more about how continuous freeze-drying enables the stabilization of your mRNA therapy for distribution and storage.
For formulation development, the freeze-drying process development, and production, we can be your partner in getting your therapy fast to the market with the highest quality while avoiding cold chain logistics.
For the most up to date news, please follow us on LinkedIn via the link below
New poster publications available
Publications
For the most up to date news, please follow us on LinkedIn via the link below
Condensorless freeze-drying
Publications

Presented poster on freeze-drying as a gene therapy stabilizer
Easier in maintenance, less complicated CIP/SIP procedures and shorter change over times.
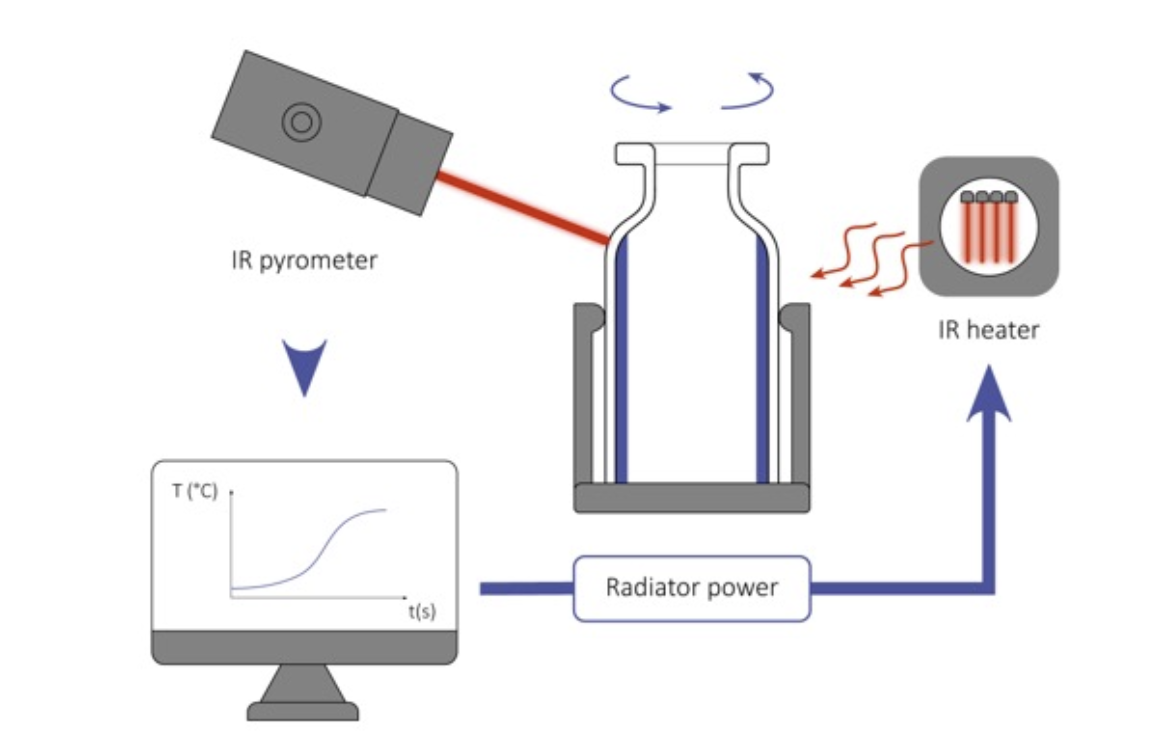
Condensorless freeze-drying has a lot of advantages. RheaVita’s continuous freeze-drying technology combined with Edwards Vacuum vacuum pumps makes it possible.
Read all about it in this white paper which you can read and download for free this page on our website.
Do you have questions? Don’t hesitate to reach out to us via info@rheavita.com
For the most up to date news, please follow us on LinkedIn via the link below