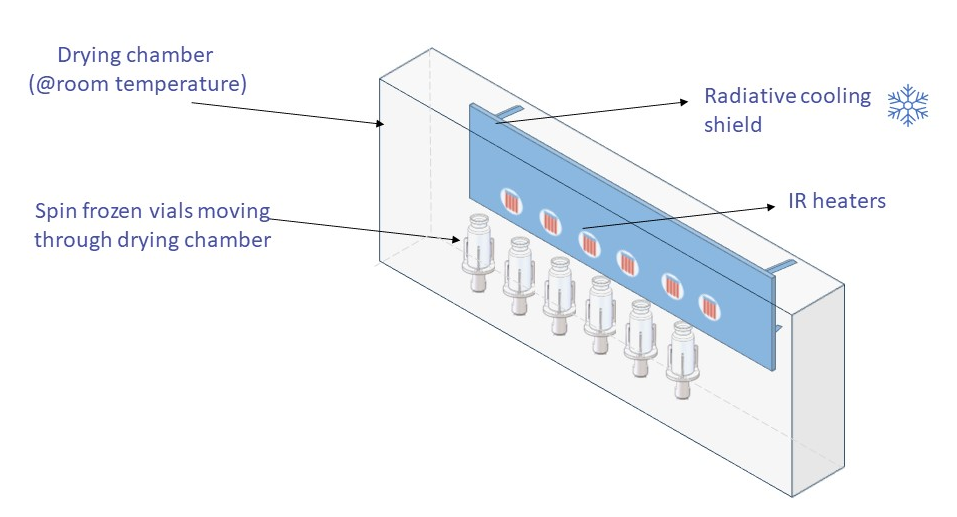
Concept
Spin freezing
During freezing, vials are rapidly rotated along their longitudinal axis while the flow of an inert and cold gas cools the drug formulation leading to the solidification of the product over the entire inner vial wall. As a result, a thin product layer with an increased area for ice sublimation is obtained, speeding up the drying process and allowing the continuous processing of these vials.
Continuous drying
Efficient and uniform drying of the spin frozen vials is obtained by applying non-contact radiative energy transfer towards the complete vial surface. For each spin frozen vial there is an individual infrared heater allowing to control and optimize the entire drying trajectory.
Model-based design and control
- Process developed and designed for purpose based on first principle understanding
- White box process
- Allows fast model based formulation and process development (i.e., optimized product quality and process efficiency) with very limited material consumption, providing important shorter time-to-market opportunities
- Allows design space determination with risk control
- Essential for optimal process monitoring, process control & real-time release
- Digital twin: support and substantiate design and engineering decisions
- A software tool is developed and available based on first principle process models, supporting fast formulation and process development with very limited material consumption.
Radiative Cooling Shield
Once primary drying is finished, the product temperature is gradually increased to facilitate the desorption of residual moisture. During this temperature ramp, it is important to maintain the product temperature below the glass transition temperature (Tg) of the dried product. The Tg is characteristic for each amorphous product and changes with the residual moisture. The slope at which the temperature increases depends on the radiative heat transfer to the vial. The background radiation of the walls of the drying unit brings a lower limit on this slope. To eliminate this background radiation, a Radiative Cooling Unit has been developed that surrounds the vial and can be cooled. This cooling shield allows the temperature ramp from primary to secondary drying to be set at the desired slope, with a minimum of 0.1 K/min.
This cooling shield also allows the expansion of the design space during primary drying. Products characterized by a very low glass transition temperature of the freeze-concentrated formulation (Tg’) require a very low radiative heat transfer during sublimation to avoid cake collapse. By eliminating the background radiation of the drying unit, these products can also be freeze-dried under optimal conditions