
Our technology
Spin-freezing
The RheaLyo™ technology is based on spinning the vials while cooling and freezing in a controlled way using cold gas. This results in a thin product layer spread over the inner vial wall. The frozen vials are subsequently dried under vacuum, where individual infrared heaters provide the radiative energy transfer for fast sublimation and desorption.
During freezing, vials are rapidly rotated along their longitudinal axis while the flow of a sterile, cold gas cools the formulation along the entire inner vial wall. As a result, a thin product layer with a large surface area is obtained. Hence a rapid drying process of a couple of hours is achievable.

Process Analytical Technologies (PAT)
Monitoring and control of critical process and product parameters at the level of each individual vial are an essential part of the RheaVita continuous freeze-drying technology.
Process Analytical Technologies (PAT) for process monitoring and control of critical process and product parameters at the level of each individual vial are an essential part of the RheaVita continuous freeze-drying technology. Several tools, such as near-infrared (NIR) Spectroscopy and thermal imaging can be included. NIR spectroscopy provides detailed in-line information about several quality attributes like residual moisture content, protein conformation and the solid state of the product. It may allow for the accurate detection of secondary drying endpoint.

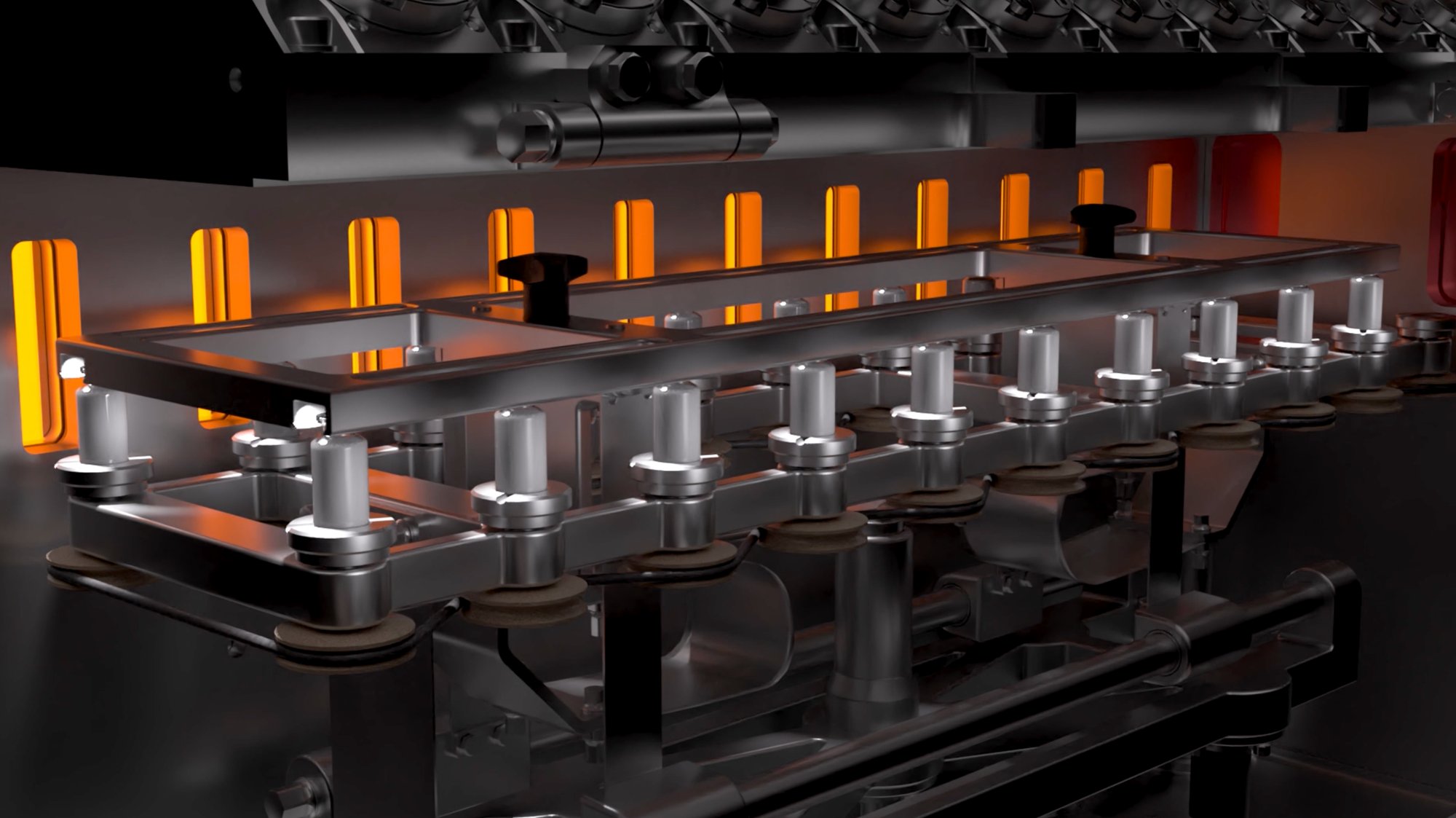
Continuous lyophilization
Frozen vials move in a stepwise fashion from a dedicated controlled infrared heater to the next one. Smart closed loop PID algorithms ensure an optimized drying profile never exceeding a pre-defined critical temperature.
Efficient and uniform drying of the frozen vials is obtained by applying contactless radiative energy transfer towards the vial surface. For each frozen vial there is a dedicated controlled infrared heater ensuring an optimized temperature profile.
Frozen vials move in a stepwise fashion from station to station receiving energy from dedicated controlled infrared heaters. Smart closed loop PID algorithms ensure an optimized drying profile.
Technology access
Feasibility Studies: Trust our team to freeze-dry your formulation with the RheaLyo™ technology. No CAPEX investment needed. Our most economical option to explore the technology.
RheaLyo™ Mono purchase / Rent: Own a RheaLyo™ Mono freeze-dryer and conduct the experiments as you like on your formulations without any IP concerns. For focused, short projects it can be more attractive to rent the device.
Production runs on our GMP-Flex “2/44” line: Outsource the production of TOX and stability batches. Process parameters are identical to future clinical and commercial batches.
GMP-Flex line purchase: Initiate a Pre-Engineering Project with RheaVita and obtain all the necessary information for the future implementation of a RheaLyo™ GMP-Flex line in your facility.
Outsourcing to your preferred C(D)MO: If you prefer to work with a C(D)MO, but the organization is not familiar with the RheaLyo™ technology, just let us know. We provide a free consultancy to educate your partner.










